RPD
35 years of experience
dedicated to microbiology
CONCEPT
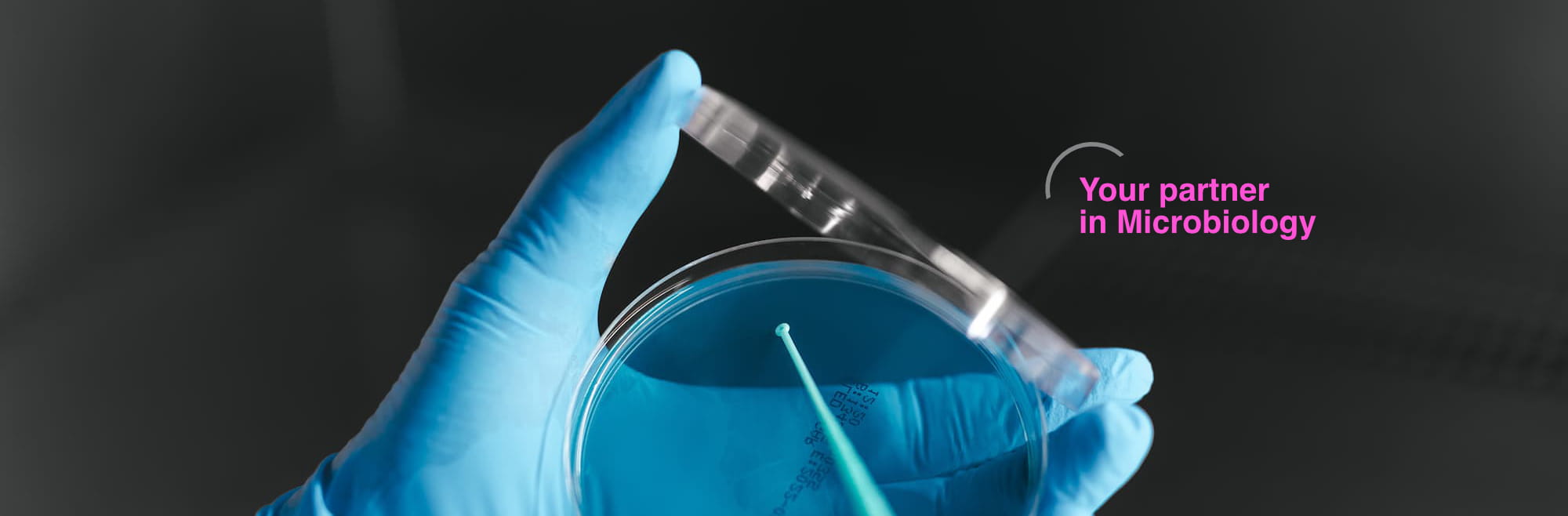
From microbiologists for microbiologists
REACTIVOS
PARA
DIAGNÓSTICO
From Microbiologists for Microbiologists
Company dedicated to the production (with its own brand or in OEM contract) of culture media for microbiology, supplements and additives for the quality control, research and production laboratory.
As a second line of business, it designs and builds equipment for the industrial production of prepared culture media, from plate and tube fillers to media preparers.
CLIENTS
Access our Customer Portal
A platform for managing your quality certifications.
HISTORY
35 years of experience dedicated to microbiology
The beginnings of Reactivos Para Diagnóstico S.L. date back to 1995, when Daniel Asensio started a project to manufacture culture media for microbiological analysis, in a 100m2 premises located in Hospitalet de Llobregat, Barcelona.
From the beginning, the essence of the business is focused exclusively on the manufacture of the culture media, and creates a business model based on manufacturing under O.E.M. conditions, for which agreements are reached with different commercial brands of recognition in the sector (both national and international).
SERVICES
Our Goal, Quality and Service
At RPD we have a wide range of services and products: From standard means in accordance with regulations, to means tailor-made to fit the needs of our customers.
We offer specialized solutions in microbiology for various sectors, guaranteeing accurate, reliable analyses under the highest quality standards.
QUALITY
One of RPD’s main bets
It has always been the quality of our products.
With a rigorous raw material selection process, a constant focus on continuous improvement of both product and technology, always committed to the quality of the products made, RPD has two ISO certifications: ISO9001 and ISO 13485 and manufacturing processes under GMP guidelines.
TEAM
The talent and passion that drive our project
We have a team of highly qualified professionals committed to research, innovation and quality in the field of microbiology. Each member of our team brings their experience and expertise, working collaboratively to deliver effective and accurate solutions.